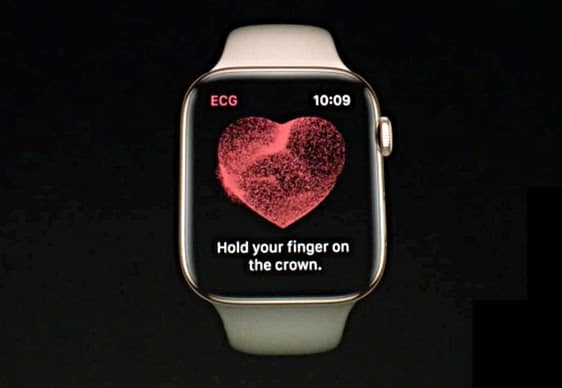
One of the Apple Watch Series 4’s massive new features is its electrocardiogram (EKG/ECG) heart rate monitor.
The device is capable of monitoring irregular heart rhythms and reportedly using this to identify potential episodes of atrial fibrillation, a.k.a. AFib. As exciting as the FDA-cleared technology sounds, however, some have expressed their concerns about the tech. A new report cites several concerns — including about Apple’s usual levels of hyperbole.
What data was required for FDA clearance?
The article in question comes from the website HealthNewsReview.org. It digs into the question of what data exactly led to the FDA giving Apple clearance (don’t call it approval) for its new wearable.
While the accuracy reported — based on one data set of 588 people and another of 226 — certainly sounds impressive, it notes that, “neither of these sets of data have been published or peer-reviewed, a point that wasn’t made in any of the news coverage.”
In both of these data sets, the researchers analyzing the data also already knew who had AFib.
Concerns going forward
The article goes on to quote University of Michigan cardiologist Venkatesh Murthy, who says that these early numbers will undoubtedly change when it is tested on a larger population of people.
“The big problem with this conclusion is that this population has a prevalence of AFib that is probably 100-fold larger than Apple’s target market,” Murthy said. “This is not good. However, the major caveat here is that we are still lacking most of the information needed to be sure how this experiment was done, so we really are just guessing.”
Murthy also questions the apparent stamp of approval the American Heart Association (AHA) has given the Apple Watch Series 4 by having president Ivor Benjamin appear on stage at last week’s Apple event.
“It is unclear whether the AHA had any specific opportunity to review the data or subject it to a peer-review process before endorsing,” he said. “The nature of the endorsement really was without recent precedent as best as I can recall.”
The ultimate guardian for your health?
The problem here isn’t necessarily with the technology itself — which may (and probably does) work incredibly well. Hence the FDA clearance.
It is, instead, the risk of misleading the public into thinking that the device can be totally relied on it and is, to use the words of Apple COO Jeff Williams, the “ultimate guardian for your health.” Apple is a brilliant company when it comes to marketing. As a recent video compiling all the adjectives at last week’s Apple event makes clear, it is also a master of hyperbole. But when it comes to selling medical products, rather than just cool consumer products, this behavior won’t necessarily fly.
As Murthy concludes, “We’d never accept such broad statements from a pharmaceutical ad and we shouldn’t accept it from marketing for a device.”